carboxylate ir stretch
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and.

Ir Spectra Of Indole 3 Acetic Acid In Kbr Indole Nh Band At 3389 Cm 1 Download Scientific Diagram
Gas-phase spectra of a series of benzoate anions have been recorded and compared to condensed-phase spectra revealing the profound influence of the environment on the symmetric and antisymmetric carboxylate stretch modes.

. We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate.
In the case of the ceC-H bond carbon is slightly negatively and hydrogen slightly positively polarized. Table of IR Absorptions. As in ketones if the carbons adjacent to the aldehyde group are unsaturated this vibration is shifted to lower wavenumbers 1710-1685 cm -1.
This attribute is why the pair of carboxylate stretching peaks are so intense. The infrared spectra of six aqueous carboxylate anions have been calculated at the m05-2xcc-pvtz level of theory with the smd solvent model and validated against experimental data from the literature over the region of 1700 cm -1 to 1250 cm -1. In theory one could identify the metal ion.
Lets compare the strength of that bond to a carbon-hydrogen bond where the carbon is SP3 hybridized. When the bonds of the -CO 2 group stretch asymmetrically and symmetrically there is a large value of dµdx. Thus ketones are said to inhabit the range of 1715-1740 cm -1 and simple esters come at 1740-1760 cm -1 some 20-30 cm.
Furthermore we have shown that these shifts in wavenumber occur primarily due to how bonding with the metal changes the carboxylate CO bond lengths and OCO angle. A bond vibration like stretching will only be IR-active ie. Weatherability of pastel and dark colored rigid PVC compound can be achieved by substituting the more light.
This region corresponds to the stretching modes of the carboxylate group and is often. Absorption peaks above 3000 cm -1 are frequently diagnostic of unsaturation. Primary amines produce two N-H stretch.
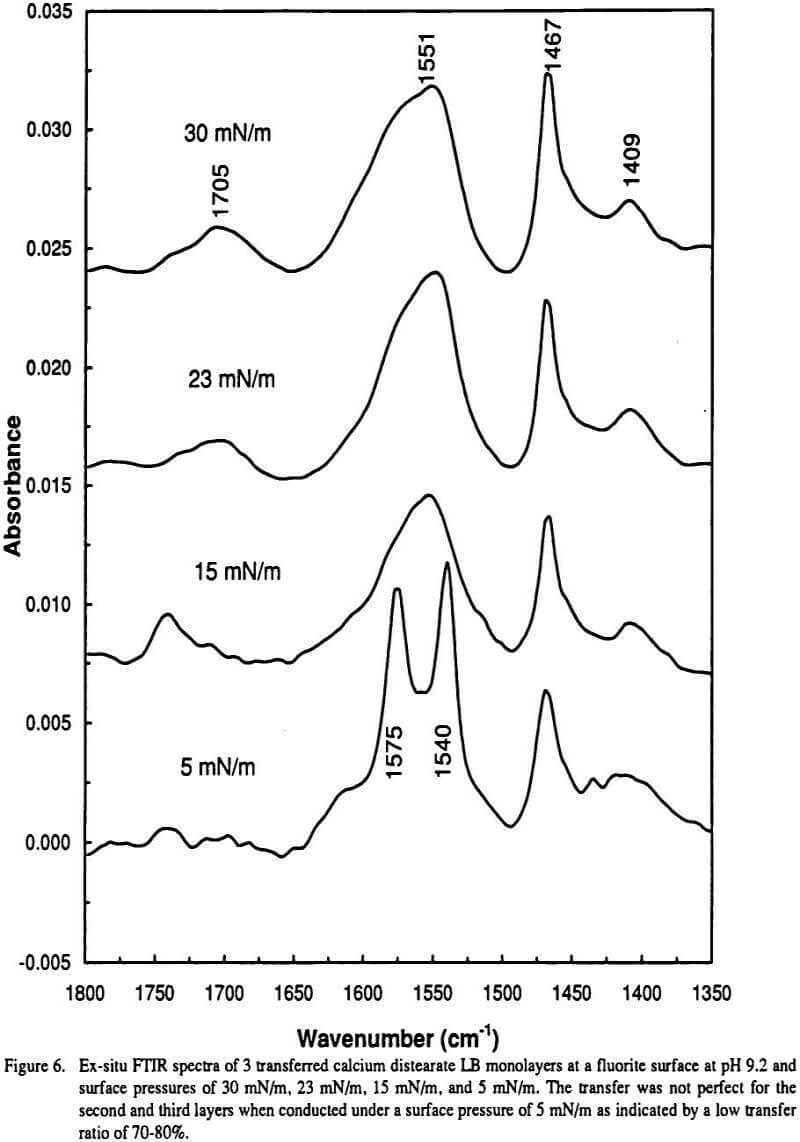
Carboxylate anions exhibit characteristic vibrational spectra in the infrared IR which typically includes an intense peak due to symmetric stretching of the COO functional group as well as a peak due to antisymmetric stretching. The carbonyl stretch CO of saturated aliphatic aldehydes appears from 1740-1720 cm -1. Infrared monolayers spectra of the metal-carboxylate complexes and the wavenumbers of the symmetric and antisymmetric vibrational modes are reported.
Alkane C-H bonds are fairly ubiquitous and therefore usually less useful in determining structure. We know that the wave number is dependent on two things from an earlier video. Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate.
Hyrdogen-Bonded Hydroxyl Groups in the Introduction to IR Spectra for more information. This is the bond stretch for nitrogen-hydrogen so thats this bond stretching right there. The analysis is performed by assigning the value of the CO stretching wavenumber to a particular range characteristic of each type of compound.
Give a band in the IR spectrum if it is accompanied by a change of dipole moment. Spectra were recorded in regions below 800 cm 1 8001500 cm 1 the fingerprint region the region between 2800 and 3000 cm 1 C-H stretch region and finally the region between 3000 and 3600 cm 1 O-H stretch region by method as mentioned by Belton et al. So in magenta this is the nitrogen-hydrogen bond stretch.
We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. With respect to metalcarboxylate interactions the most useful characteristic bands obtained by FTIR are a strong asymmetric COO stretching vibration ie νasym COO and a somewhat weaker symmetric COO stretching vibration ie νsym COO.
21 3 Spectroscopy Of Carboxylic Acids Chemistry Libretexts
Ir Infrared Absorption Bands Of Carboxylate

Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

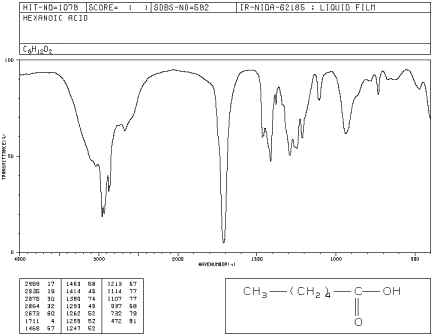
Infrared Spectrum Of Butanoic Acid C4h8o2 Ch3ch2ch2cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Butyric Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
The C O Bond Part Iii Carboxylic Acids

Lec15 Ir Spectra Of Carboxylic Acids Amides And Esters Youtube

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Interpretation Of Carboxylic Acid Ir Spectra Youtube
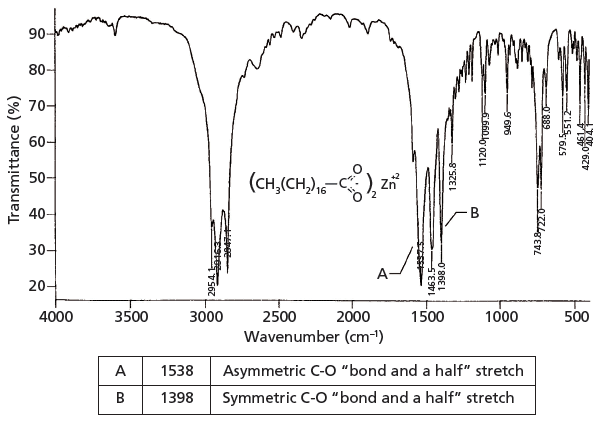
The Carbonyl Group Part V Carboxylates Coming Clean
The C O Bond Part Iii Carboxylic Acids
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate
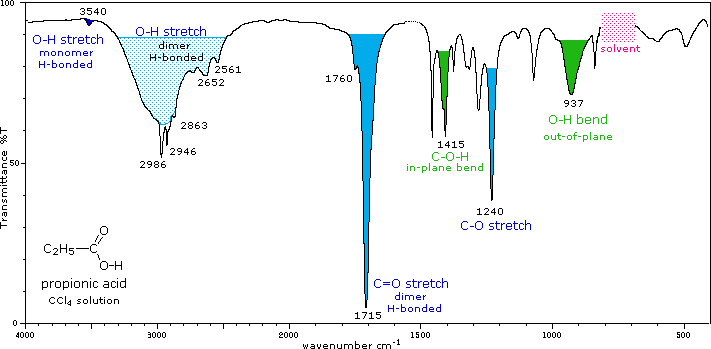
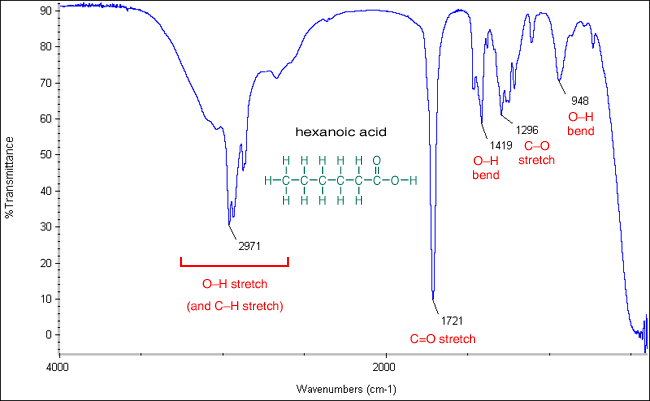
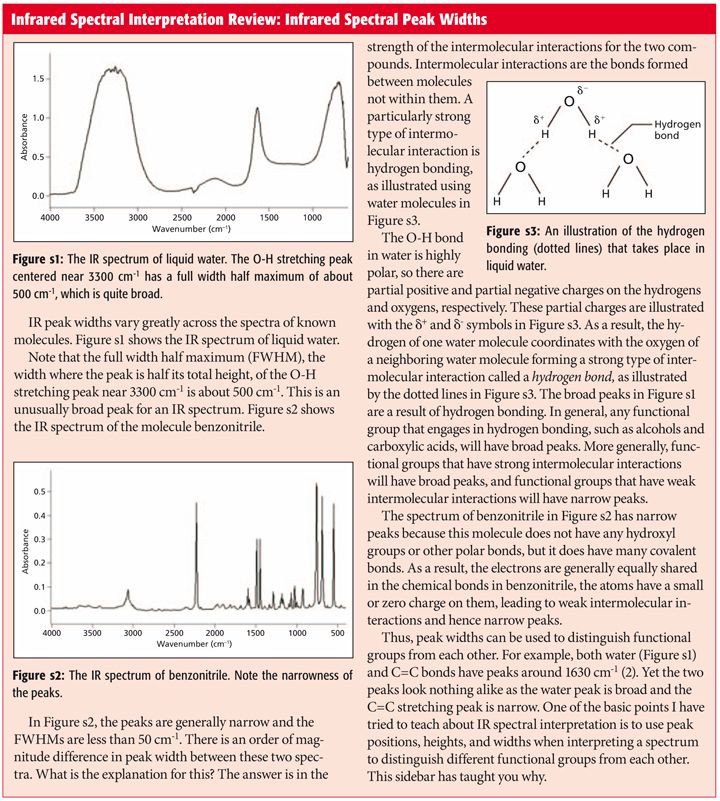
Comments
Post a Comment